Orchestrating gut health™
The future of health is in your gut. EnteroBiotix is pioneering this future
Welcome to the future
of gut health
We’re a specialist pharmaceutical company focused on gut therapeutics
OUR VISION
To be the leader in developing orally administered therapies that harness the full potential of gut ecology to transform patients lives
EnteroBiotix® at a glance
Company summary
Privately-held, clinical-stage biotechnology company focussed on full-spectrum microbiome therapeutics
Head-quartered in Scotland with 50 employees
Raised £47m in equity investment to build platform and deliver clinical data in two high-value indications
Clinical pipeline
Focus: high-value indications linked to microbiome-related impaired gut function
Completed Ph1b in liver cirrhosis with promising data
Ongoing Ph2 trial in IBS-C/D
Ongoing partnered Ph2a clinical trial with Imperial College in Allo-HSCT patients
Core technology & strengths
Innovative stabilising technology for anaerobic microorganisms, delivering ambient temperature stability and diversity
Control over the supply chain, ability to meet and exceed demands
Sophisticated analytical toolkit for precise microbial characterisation
Our approach
Full-spectrum
gut therapeutics
Our pioneering Intestinal Microbial Transfer (IMT) therapies are preventing and treating disease, through the restoration and enhancement of gut microbial ecology
Rebuilding the foundation
of gut health
Not just treating symptoms
Restoring the gut’s foundation by rapidly addressing the balance and function of the microbiome
Full-spectrum microbial ecosystems
Products utilise microorganisms sourced from exceptionally healthy donors with desirable characteristics
Consistent, stable, high-diversity anaerobic microbiota
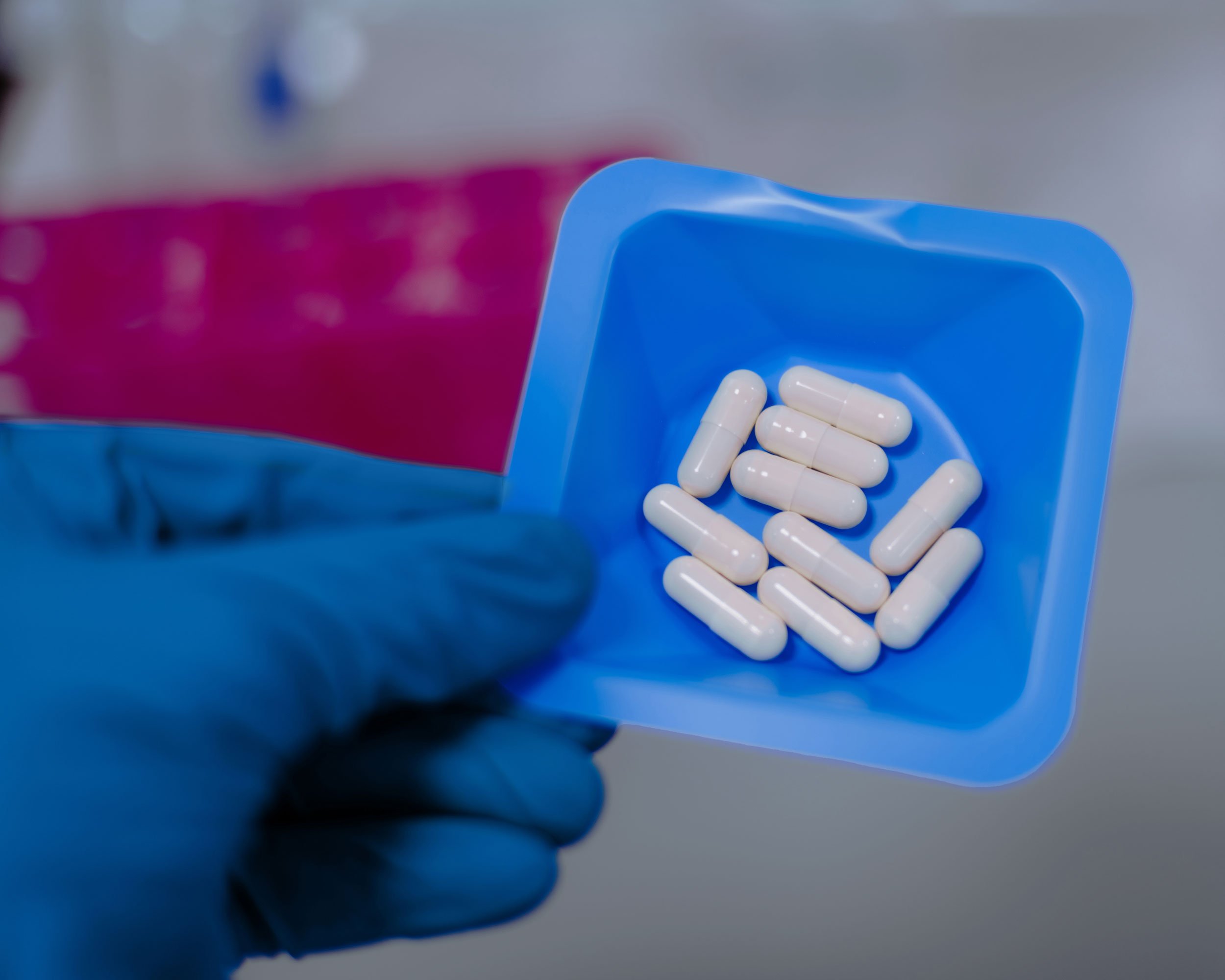
Unique powder formulation
An off-white, odourless and stable oral capsule, delivering high-diversity anaerobic microbiota. Inter-donor pooling and starting material controls delivers consistency and safety
Beyond limited strains
In contrast to limited defined consortia, the EnteroBiotix approach embraces the full diversity of the microbiome. This ensures a more adaptable, resilient ecosystem that naturally supports a wide range of gut functions without the limitations of narrowly focused products
Promising safety and
efficacy data
Naturally found in healthy humans
Drugs are based on microbes naturally found within the healthy human gut. Available placebo-controlled data suggests drug is safe and well-tolerated
Efficacy signals
Ph1b data in cirrhosis reports that drugs improve local and systemic outcomes, such as gut barrier function and hospital anxiety and depressions scores. Ph2 IBS-C cohort interim analysis has generated efficacy signals
Next-generation platform enables our differentiated approach
-

Number 2®
In-house donor programme
-

PRISM™
Sophisticated analytical toolkit
-

AMPLA™
Proprietary drying technology
-

Manufacturing
State-of-the-art GMP capabilities
-

Stable drug substance
Superior characteristics
-

Oral capsules
Delivered without bowel prep

EBX Newsroom
Meet our pioneering team
EBX is led by an entrepreneurial and experienced management team, supported by a distinguished Board
Join us in our mission
to orchestrate gut health
If you are passionate about improving the health of others and transforming lives, we are always looking for talented, ambitious, collaborative, and adaptable people
Partnering opportunity
We welcome partnering enquiries about our platform or our pipeline. Get in touch to find out more















